企业性质生产商
入驻年限第5年
SyncroPatch 384高通量全自动膜片钳系统
新型 SyncroPatch 384 是一种革命性的全自动膜片钳系统,由集成有先进的液体处理系统 Biomek i5 的膜片钳模块组成。 使用 384 通道放大器和一个 384通道移液头,所有384 个细胞都被并行记录,从而产生每天 20,000 个数据点的通量。得益于其易用性和开放式设计,SyncroPatch 384 支持完全自动化并集成到 HTS 环境中。
但 SyncroPatch 384 不仅仅是一个高通量筛选系统,而且可以在您的所有电生理学项目中实施,无论您的通量需求如何。 32 孔操作模式非常适合较小的筛选项目和学术研究,并充分利用了经济实惠的NPC-384芯片。 以 32 的倍数选择您需要并行记录的孔数,您可以在几天内使用剩余的孔。 或者,您可以使用 SyncroPatch 384 进行长达8 小时的无人值守模式的全自动实验。
数据质量和灵活性使 SyncroPatch 384 成为制药公司、CRO 和学术机构等需要的384通道全自动膜片钳系统。
主要特征
• 千兆级封接记录
• 384孔平行记录
• 32孔模式适用于较少的化合物筛选和研究项目
• 通常成功率达到>85%
• 用于快速脱敏配体门控离子通道的快速外液更换(高达110 µl/s)
• 记录时的內液灌流——通过內液激活通道,例如钙激活K+通道。
• 高级温度控制可以使实验过程中降低或升高的温度(范围10-37°C)标准化
• 具有电流钳功能的基本特点
• 单孔芯片用于高表达细胞系,多孔芯片用于低表达细胞系。 所有芯片都是通过室内质控生产
• 可以收集样品数据进行量效曲线分析
• 受益于良好的服务和技术支持
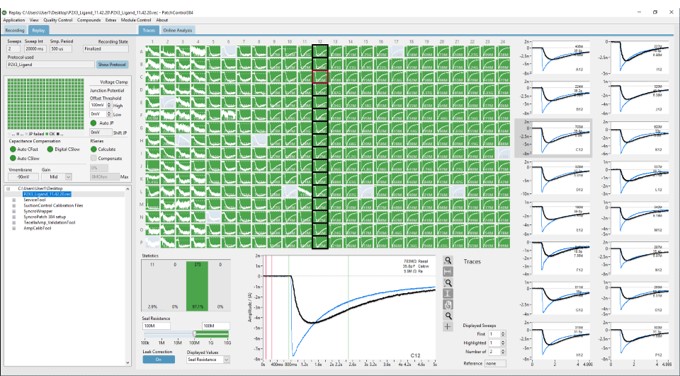
PatchControl 384
PatchControl 384是一个功能强大的图形用户界面,用于直观、快速、轻松地设置电压协议和实验参数。记录井根据用户定义的质量标准(例如密封电阻、串联电阻或电容)进行可视化和颜色编码。单击鼠标一次,视图切换到在线分析结果,例如I/V曲线或浓度响应曲线。
DataControl384:分析软件
DataControl 384用于可视化和分析PatchControl 384数据,采用用户定义的数据分析模板。结果(自动IC50、EC50、IV关系图生成)、复合信息和质量控制参数以用户定义的导出格式一起导出,自动生成pdf报告,并为进一步的数据库集成准备数据。这一过程简单、直观且快速完成。
NPC-384
NPC-384芯片是SyncroPatch 384的高性价比和高质量耗材。它在慕尼黑的Nanion总部内部生产,质量有保证。可提供不同类型的NPC-384芯片,应根据单元大小和应用选择。
带有贴片孔的硼硅酸盐玻璃载玻片封装在384孔板中,形成孔,在孔板中输送电池和外部溶液。芯片的设计允许在实验过程中灌注内部溶液。每个NPC-384芯片包含384个记录室。这些站点可以一次性使用,也可以在32井模式下使用,部分芯片可以在32的倍数下使用,其余部分可以在数天内使用,不会降低成功率。在SyncroPatch 384上可以测量一个芯片,在机器人上可以堆叠25个芯片进行无人值当实验。芯片的开放性设计使样品的采集和化合物浓度的后续验证成为可能。此外,内部或外部解决方案的交换次数是无限的。NPC-384芯片可用于GOhm密封的单井眼,也可用于单井眼,以增加测量电流幅值,提高成功率。
Available chip types
"NPC-384, 1x medium resistance": One hole per well (Order # 221102)
"NPC-384, 1x medium resistance plus": One hole per well (Order # 221104)
"NPC-384, 4x medium resistance": 4 holes per well (Order # 221402)
"NPC-384, 1x high resistance": One hole per well (Order # 221101)
"NPC-384, 4x high resistance": 4 holes per well (Order # 221401)
"NPC-384, 1x low resistance": One hole per well (Order # 221103)
"NPC-384, 4x low resistance": 4 holes per well (Order # 221403)
"NPC-384, 8x": 8 holes per well (Order # 221801)
SyncroPatch 384/384i/768i的缓冲液和解决方案
可靠的缓冲溶液对于任何电生理应用都至关重要。因此,我们的目标是提供合适的解决方案,让您对质量和稳定性毫无疑问。我们的质量保证包括化学测试以及每批膜片钳系统的测试。我们的缓冲器随附相应的“分析证书”和“材料安全数据表”(MSDS)。
Available buffers and solutions
"External Standard", 500 mL: (Order # 08 3001)
"External Standard Ca 10", 500 mL: (Order # 08 3012)
"External NMDG 60", 500 mL: (Order # 08 3004)
"External NMDG 60 Ca 10", 500 mL: (Order # 08 3011)
"External [-] Ca2+ [-] Mg2+", 500 mL: (Order # 08 3003)
"Internal CsF 110", 500 mL: (Order # 08 3008)
"Internal KF 110", 500 mL: (Order # 08 3007)
"Washing solution", 5 L: (Order # 08 3010)
数据与应用:
32- well mode for smaller screens or academic investigations SyncroPatch 384 data and applications:
SyncroPatch 384 data and applications:
Cells were kindly provided by SB Drug Discovery
An exemplary 32-well Mode Experiment. A small fraction of the chip can be used at a time, which is ideal for smaller compound screens.
Consecutive experiments of 32-wells on the same NPC-384 patch clamp chip over multiple days. Success rate and accurate pharmacology remains stable over 8 days as shown in the figure. Nav1.5 recordings in the presence of increasing Mexiletine concentrations.
AMPA Receptor (GluA2) - Activation by Glutamate
 SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by SB Drug Discovery.
The AMPA receptor (GluA2) was activated using different concentrations of glutamate (1 µM - 100 µM). Measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384), the whole cell patch methodology and multi-hole chips were used.
The lower two images are displaying screenshots of single cell currents after repetitive glutamate applications:
Left: The same concentration of Glutamate was applied three times.
Right: Four different Glutamate concentrations were applied in a cumulative manner.
AMPA Receptor (GluA2) - Cumulative Concentration Response
 SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by SB Drug Discovery.
The AMPA receptor (GluA2)was activated by increasing concentrations of glutamate on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384). L-glutamate was applied for approximately 500 ms in increasing concentrations (A) and a cumulative concentration response curve for glutamate was constructed for 222 wells (C).
The online analysis values peak amplitude and area under the curve (AUC) are shown versus time in Panel B. The fast activation of GluA2 could be captured at higher concentrations (inset; 1 mM).
AMPA Receptor (GluA2) - Pharmacology
 SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by SB Drug Discovery.
The AMPA receptor (GluA2) was analyzed using different positive and negative allosteric modulators (CNQX, LY404187, LY395153, CP465022, Cyclothiazide). After activating the receptor by application of Glutamate, the modulating compound plus glutamate was applied afterwards. Measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384), the whole cell patch methodology and multi-hole chips were used.
The lower images on the left hand side are displaying a screenshot of a current after application of the positive modulator LY404187. The EC50 was determined as 379 nM.
Cardiac Ion Channels - Pharmacology of Sotalol
 CardioExcyte 96 and SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
CardioExcyte 96 and SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River and Cellular Dynamics.
The image on the left hand side displays the results of the blocking effect of Sotalol on hERG. The result is in good agreement with manual patch clamp data (Crumb et al., 2016). The compound induced arrhythmia when iPSC-CM were exposed to a minimum concentration of 10 µM. Arrhytmic events were both detected in field potential recordings as well as in the impedance based contractility measurements.
CaV1.2 - Current Voltage Relationship

SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells kindly provided by Charles River.
CaV1.2 expressed in CHO cells recorded on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384). A The screenshot shows the data acquisition and analysis software used on the SyncroPatch 384PE. The online analysis values are shown for a current-voltage experiment. B The raw traces from an example cell elicited by depolarizing steps from -60 mV to 40 mV in 10 mV increments from a holding potential of -80 mV are shown. C The normalized current-voltage plot for an average of 272 cells. A Boltzmann equation fit revealed a V0.5 of activation of -4.8 mV
CaV1.2 - Pharmacology of Nifedipine, using the CiPA protocol
 SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software showing hCaV1.2/β2/α2δ1 current traces in response to the CiPA voltage step protocol and the corresponing current-voltage relationship plot. Measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384) using perforated patch methodology (Escin) and multi-hole chips (4 holes per well), the success rate of valuable data for the analysis was 94%. The IC50 value of Nifedipine was determined as 106 nM
CaV1.2 - Stable recording from frozen stock cells
 SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software showing hCaV1.2β2/α2δ1 current traces in response to a voltage step protocol and the corresponding current-voltage relationship plot. Measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384) using perforated patch methodology (Escin) and multi-hole chips (4 holes per well), the success rate of valuable data for the analysis was 100 %. The cells were used from a frozen cell stock (after induction) and recorded stably for more than 20 minutes. The IC50 value of Nifedipine was determined as 21 nM
ClC-1 - Current-voltage plot
 SyncroPatch 384i (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384i (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Activation of hClC-1 tail currents expressed in CHO cells recorded on the SyncroPatch 384i (a predecessor model of SyncroPatch 384). A pre-pulse voltage step to +60 mV was followed by voltage steps from -120 mV to +80 mV for 300 ms (increasing in 20 mV steps) and the tail current was measured at the subsequent step to -100 mV. Out of a possible 384 wells, all 384 wells were used for the IV analysis
ClC-1 – Inhibition by 9-AC
 SyncroPatch 384i (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384i (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Tail currents of ClC-1 expressed in CHO cells were inhibited by increasing concentration of 9-AC. A single concentration of 9-AC was added to each well and the concentration response curve constructed over multiple wells. The IC50 was calculated to be 6.3 µM for an average of 352 wells. The average current traces are also shown
GABAA Receptor (α1β2γ2) - Success Rates

SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Bsys.
Statistic of hGABAA α1β2γ2 cells recorded on one NPC-384 1-hole (1x) patch clamp chip. 57 % of the cells on one NPC-384 chip had seal resistance > 1 GOhm at the beginning and 48% at the end of the experiment. Access (RSeries) was good with 80% of cells with RSeries <20 MOhm at the start of the experiment.
GluA2 activation at 110µl/s – speed is key
 SyncroPatch 384 data and applications:
SyncroPatch 384 data and applications:
Cells were kindly provided by SB Drug Discovery
The AMPA receptor (GluA2) was activated using increasing concentrations of glutamate. Measured on the SyncroPatch 384 the whole cell patch methodology and multi-hole chips were used. The faster you apply the ligand, the shorter is the Time to Peak, this means pipetting speed is relevant for accurate pharmacology. The IC50 of Glutamate at 110 µl/s was 460 µM
Glycine Receptor (GlyRa1) - Reproducible Current Recordings
 SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Glycine-mediated current traces and corresponding time plots from 384 simultaneously recorded HEK cells are shown. Multiple additions of 50 µM Glycine produce very robust current responses with similar peaks, providing best conditions for cumulative pharmacology on one cell.
hERG - Pharmacology at Physiological Temperature using the CiPA Protocol
 SyncroPatch 384/768 PE (a predecessor model of the SyncroPatch 384) data and applications:
SyncroPatch 384/768 PE (a predecessor model of the SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software showing hERG current traces in response to the CiPA voltage step protocol at 35 degree Celsius. Measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384) using perforated patch clamp methodology (Escin) and multi-hole chips (4 holes per well). The IC50 value of Erythromycin of the peak current was determined as 60.5 µM.
hERG - Pharmacology using the CiPA Protocol
 SyncroPatch 384/768 PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384/768 PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software showing hERG current traces in response to the CiPA voltage step protocol. Measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384) using whole cell patch clamp methodology and multi-hole chips (4 holes per well). The IC50 value of the following compounds of the peak current was determined as 4.18 µM for Diltiazem, 37.4 nM for Terfenadine, 971 nM for Quinidine, 63 µM for Mexiletine, 431 nM for Verapamil and 4.54 µM for Ranolazine.
hERG - recordings with great stability using the CiPA step ramp protocol
 SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software showing hERG current traces in response to the CiPA voltage step protocol. Measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384) using perforated patch clamp methodology (Escin) and multi-hole chips (4 holes per well).
hERG - Stable Recordings with Accurate Pharmacology
 SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River Laboratories.
Current-voltage relationship of hERG (Kv11.1) expressed in HEK293 is shown along with pharmacology of 4 hERG-active compounds. The current-voltage relationships for all 384 wells (top) using perforated patch (Escin) and multi-hole chips (4 holes per well) are shown. In all 384 wells, a hERG-mediated current was observed with peak amplitude >700 pA at -20 mV. Using a pharmacology voltage protocol, experiments were stable lasting over 20 minutes. Concentration response curves for astemizole, pimozide, cisapride and terfenadine revealed IC50 values consistent with those found in the literature.
hERG and Temperature Control
 SyncroPatch 384 data and applications:
SyncroPatch 384 data and applications:
Cells were kindly provided by Charles River Chantest
Cardiac ion channels are recommended to be recorded at Phys. Temp. (ICH S7B Q&A. 2021). On the SyncroPatch 384, measurement site, cells and solutions can be accurately temperature controlled – in the presence of physiological temperatures the hERG current kinetic is changed to a larger slope and higher amplitude.
KCa1.1 (BK) - High throughput study
 SyncroPatch 384i (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384i (a predecessor model of SyncroPatch 384) data and applications:
Data kindly provided by Sharan R. Srinivasan1 and Vikram G. Shakkottai1,2
1Department of Neurology, University of Michigan, Ann Arbor, MI 48109;
2Molecular and Integrative Physiology, University of Michigan, Ann Arbor, MI 48109.
HEK293 cells stably transfected with BK channels were used to screen over 50,000 compounds, and using clever buffering techniques, targeting only activators of calcium sensitivity for BK channel augmentation.
KCa3.1 (SK4) - Activation by Perfusion of free internal Calcium
 SyncroPatch 384/768 PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384/768 PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software are showing KCa3.1 raw traces and according time plots (online analysis) to a voltage ramp from -120 mV to + 60 mV over 200 ms. The application of internal Ca2+ is indicated by the yellow bar. The current increased upon application of internal Ca2+ reaching a peak within 1-2 min after the start of the perfusion. Five minutes of stable KCa3.1 current was recorded prior the channel was inhibited by cumulative additions of external Ba2+; first partly (1 mM Ba2+) and then completely (5 mM Ba2+). The recording was performed with perfectly high success rates in whole cell configuration on a multi hole chip (4 holes per well) using the SyncroPatch 384PE (a predecessor model of SyncroPatch 384)
Kir2.1 - Pharmacology of Barium
 SyncroPatch 384/768 PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384/768 PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software showing Kir2.1 current traces in response to a voltage step protocol. Measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384) using the whole cell patch methodology and multi-hole chips (4 holes per well), the success rate of valuable data for the analysis was 93%. The IC50 value of Barium was determined as 6.38 µM (Literature: 16.2 µM, Schram et al. Cardiovasc Res. 2003)
KV1.3 - Pharmacology with High Success Rate
 SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Evotec.
Shown are screenshots of a pharmacology experiment performed with the SyncroPatch 384PE (a predecessor model of SyncroPatch 384). Recordings from 384 KV1.3 expressing CHO cells were performed simultaneously. Original current traces and the peak current over time are displayed. Data are analysed with DataControl384 full analysis tool. With just a few mouse-clicks normalized concentration response curves can be generated. Here, normalized response and the IC50 of Quinidine is shown. Darkening shades of blue indicate increasing compound concentration
KV1.5 - Dose response curve of 4-AP
 SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software showing a dose-response curve of 4-AP on KV1.5 stably transfected cells. Measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384) using multi-hole chips (4 holes per well), the success rate of completed exeriments was 100%. The IC50 value of 160 µM corresponds well to literature (IC50 4-AP: 270 µM; Gutman et al., Pharmacological Reviews 57: 473-508, 2005).
KV4.3 - Pharmacology of Metropolol Tartrate, using the CiPA Protocol
 SyncroPatch 384/768 PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384/768 PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software showing KV4.3 current traces in response to the CiPA voltage step protocol, measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384) using the whole cell patch methodology and single-hole chips. The IC50 value of Metropolol Tartrate was determined as 128 µM.
KV4.3 - Pharmacology of Quinidine
 SyncroPatch 384/768 PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384/768 PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software showing KV4.3 current traces in response to the CiPA voltage step protocol. Measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384) using the whole cell patch methodology and multi-hole chips (4 holes per well), the success rate of completed experiments was 95.3%. The IC50 value of Quinidine was determined as 21.2 µM (Literature: 79.3 µM, Crumb et al., J Pharmacol Toxicol Methods. 2016).
KV4.3/KChIP2 - Dose-response curve of Flecanaide
 SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software showing KV4.3/KChIP2 current traces in response to a voltage step protocol and the corresponding current-voltage relationship plot. Using whole cell mode in combination with multi-hole chips (4 holes per well), stably transfected cells were measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384). The IC50 value of flecainide was determined as 28.3 µM which is in accordance to literature. The success rate for completed experiments was 100%.
KV7.1 (KVLQT) - Dose-response curve
 SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software showing KV7.1/KCNE (KVLQT/minK) current traces in response to a voltage step protocol and the corresponding current-voltage relationship plot. Using the perforated patch methodology (Escin) in combination with multi-hole chips (4 holes per well), stably transfected cells were measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384). The IC50 value of Chromanol 293B was determined as 3.82 µM. The success rate of completed exeriments was 100%.
NaV1.5 - Late Current Analysis using the CiPA Protocol
 SyncroPatch 384/768 PE (a predecessor model of the SyncroPatch 384) data and applications:
SyncroPatch 384/768 PE (a predecessor model of the SyncroPatch 384) data and applications:
Cells were kindly provided by Charles River.
Screenshots of the PatchControl 384 software showing NaV1.5 current traces in response to the CiPA voltage step protocol, measured on the SyncroPatch 384PE (a predecessor model of SyncroPatch 384) using whole cell patch clamp methodology and single-hole chips. The NaV1.5 late current was activated by the application of 60 nM ATX-II. The IC50 value of Ranolazine of the late sodium current current was determined as 40.4 µM
Piezo1 in Neuro2A cells - activation by Yoda1
 SyncroPatch 384PE (a predecessor model of the SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of the SyncroPatch 384) data and applications:
Piezo1 channels endogenously expressed in Neuro2A cells were investigated on the SyncroPatch 384PE (a predecessor model of the SyncroPatch 384). A Screenshot of the PatchControl 384 software during an experiment. B Statistical analysis of the currents at -100mV (left) and at 80 mV (right). 140 out of 384 Neuro2A cells (37%) passed the quality criteria and 85 cells (60% of the valid cells) were considered as Yoda1 responders.
Piezo1 in red blood cells - activation by Yoda1
 SyncroPatch 384PE (a predecessor model of the SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of the SyncroPatch 384) data and applications:
Current response of Piezo1 activated by Yoda1 in patient cells with the novel PIEZO1 mutation (R2110W) compared to healthy red blood cells (RBCs). Shown are raw data traces (top) and statistical analysis of all measured cells, independent of their response to Yoda1 (bottom).
Piezo1 in red blood cells - Hereditary Xerocytosis
 SyncroPatch 384PE (a predecessor model of the SyncroPatch 384) data and applications:
SyncroPatch 384PE (a predecessor model of the SyncroPatch 384) data and applications:
Whole-cell recordings of ion currents from RBCs of healthy donors and Hereditary Xerocytosis patients. Different mutations in the PIEZO1 gene were compared with controls. Aa The P50.2 mutation resulted in current conductance that was unchanged compared with transport controls, but showed increased conductance compared with general controls (Ab). The mutation P52.1 showed decreased conductance compared with transportation controls (Ba) and general controls (Bb).
TRPM8 and Temperature Control
 SyncroPatch 384 data and applications:
SyncroPatch 384 data and applications:
Cells were kindly provided by Charles River Chantest
At RT, the TRPM8 current was activated using increasing concentrations of Menthol (left). Measurement site, cells and solutions can be accurately temperature controlled – in the presence of temperatures 38°C, 25°C, 18°C and 12 °C the current size of temperature regulated TRPM8 changes accordingly (right). TRPM8 is activated at temperatures < 25°C.
发表文献:
2021 - The Schizophrenia Variant V1282F in SCN2A Causes Functional Impairment of NaV1.2
2021 - The insecticide deltamethrin enhances sodium channel slow inactivation of human Nav1.9, Nav1.8 and Nav1.7
2021 - Neurogranin, Encoded by the Schizophrenia Risk Gene NRGN, Bidirectionally Modulates Synaptic Plasticity via Calmodulin-Dependent Regulation of the Neuronal Phosphoproteome
2021 - Mechanism of hERG inhibition by gating-modifier toxin, APETx1, deduced by functional characterization
2021 - High-throughput characterization of photocrosslinker-bearing ion channel variants to map residues critical for function and pharmacology2021 - Heterozygous KCNH2 variant phenotyping using Flp-In HEK293 and high-throughput automated patch clamp electrophysiology
2021 - From High-Throughput Screening to Target Validation: Benzo[d]isothiazoles as Potent and Selective Agonists of Human Transient Receptor Potential Cation Channel Subfamily M Member 5 Possessing In Vivo Gastrointestinal Prokinetic Activity in Rodents
2021 - Fluorescent- and tagged-protoxin II peptides: potent markers of the Nav1.7 channel pain target
2021 - Dyshomeostatic modulation of Ca2+-activated K+ channels in a human neuronal model of KCNQ2 encephalopathy (2)
2021 - Disease-linked super-trafficking of a potassium channel2021 - Differential contributions of M1 and pre-M1 to ion selectivity in ASICs and ENaCs2021 - Comprehensive preclinical evaluation of how cardiac safety profiles of potential COVID-19 drugs are modified by disease associated factors
2021 - Cation and anion channelrhodopsins: Sequence motifs and taxonomic distribution
2021 - Applying the CiPA Approach to Evaluate Cardiac Proarrhythmia Risk of some Antimalarials Used Off‐label in the First Wave of COVID‐19
2021 - A Massively Parallel Trafficking Assay Accurately Predicts Loss of Channel Function in KCNH2 Variants
