企业性质生产商
入驻年限第9年
| 适用环境: | 野外 |
FluorPen手持式叶绿素荧光仪
 FluorPen FP110手持式叶绿素荧光仪用于实验室、温室和野外快速测量植物叶绿素荧光参数,具有便携性强、精确度高、性价比高等特点;双键操作,具图形显示屏,内置锂电和数据存储,广泛应用于研究植物的光合作用、胁迫监测、除草剂检测或突变体筛选,还可用于生态毒理的生物检测,如通过不同植物对土壤或水质污染的叶绿素荧光响应,找出敏感植物作为生物传感器用于生物检测。FP110配备多种叶夹型号,用于不同的样品与研究。
FluorPen FP110手持式叶绿素荧光仪用于实验室、温室和野外快速测量植物叶绿素荧光参数,具有便携性强、精确度高、性价比高等特点;双键操作,具图形显示屏,内置锂电和数据存储,广泛应用于研究植物的光合作用、胁迫监测、除草剂检测或突变体筛选,还可用于生态毒理的生物检测,如通过不同植物对土壤或水质污染的叶绿素荧光响应,找出敏感植物作为生物传感器用于生物检测。FP110配备多种叶夹型号,用于不同的样品与研究。
应用领域
适用于光合作用研究和教学,植物及分子生物学研究,农业、林业,生物技术领域等。研究内容涉及光合活性、胁迫响应、农药药效测试、突变筛选等。
·植物光合特性研究
·光合突变体筛选与表型研究
·生物和非生物胁迫的检测
·植物抗胁迫能力或者易感性研究
·农业和林业育种、病害检测、长势与产量评估
·除草剂检测
教学
功能特点:
§结构紧凑、便携性强,LED光源、检测器、控制单元集成于仅手机大小的仪器内,重量仅188g
§功能强大,是叶绿素荧光技术的高端结晶产品,具备了大型荧光仪的所有功能,可以测量所有叶绿素荧光参数
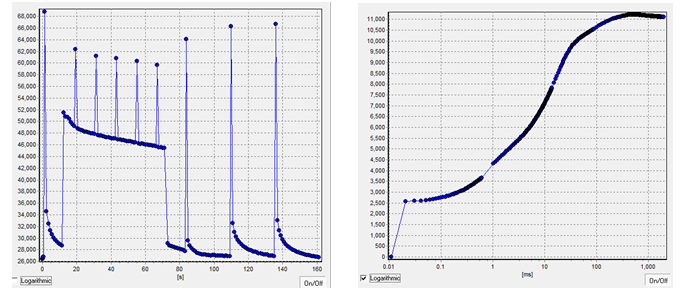
§内置了所有通用叶绿素荧光分析实验程序,包括3套荧光淬灭分析程序、3套光响应曲线程序、OJIP快速荧光动力学曲线等
§高时间分辨率,可达10万次每秒,自动绘出OJIP曲线并给出26个OJIP–test参数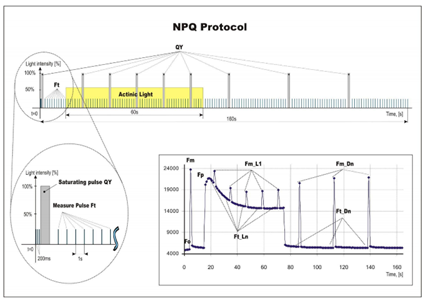
§FluorPen专业软件功能强大,可下载、展示叶绿素荧光参数图表,也可以通过软件直接控制仪器进行测量
§具备无人值守自动监测功能
§内置蓝牙与USB双通讯模块,GPS模块,输出带时间戳和地理位置的叶绿素荧光参数图表
§配备多种叶夹型号:固定叶夹式(适于实验室内暗适应或夜间快速测量)、分离叶夹式(适用于野外暗适应测量)、探头式(透明光纤探头,用于非接触性测量监测或光适应条件下的叶绿素荧光监测)、用户定制式等
§可选配野外自动监测式荧光仪,防水防尘设计
测量程序与功能
·Ft:瞬时叶绿素荧光,暗适应完成后Ft=F0
·QY:量子产额,表示光系统II 的效率,等于Fv/Fm(暗适应状态)或ΦPSII (光适应状态)。
·OJIP:快速荧光动力学曲线,用于研究植物暗适应后的快速荧光动态变化
·NPQ:荧光淬灭动力学曲线,用于研究植物从暗适应到光适应状态的荧光淬灭变化过程。
·LC:光响应曲线,用于研究植物对不同光强的荧光淬灭反应。
·PAR:光合有效辐射,测量环境中植物生长可以利用的400-700nm实际光强(限PAR型号)。
技术参数
测量参数包括F0、Ft、Fm、Fm’、QY、QY_Ln、QY_Dn、NPQ、Qp、Rfd、PAR(限PAR型号)、Area、Mo、Sm、PI、ABS/RC等50多个叶绿素荧光参数,及3种给光程序的光响应曲线、3种荧光淬灭曲线、OJIP曲线等
OJIP–test时间分辨率为10µs(每秒10万次),给出OJIP曲线和26个参数,包括F0、Fj、Fi、Fm、Fv、Vj、Vi、Fm/F0、Fv/F0、Fv/Fm、Mo、Area、Fix Area、Sm、Ss、N、Phi_Po、Psi_o、Phi_Eo、Phi–Do、Phi_Pav、PI_Abs、ABS/RC、TRo/RC、ETo/RC、DIo/RC等
测量程序:Ft、QY、OJIP、NPQ1、NPQ2、NPQ3、LC1、LC2、LC3、PAR(限PAR型号)、Multi无人值守自动监测
叶夹类型:FP110/S固定叶夹式、FP110/D分离叶夹式、FP110/P探头式、FP110/X用户定制式

PAR传感器(限PAR型号):80º入射角余弦校正,读数单位µmol(photons)/m².s,可显示读数,检测范围400-700 nm
测量光:每测量脉冲ZG0.09µmol(photons)/m².s,10-100%可调
光化学光:10-1000µmol(photons)/m².s可调
饱和光:ZG3000µmol(photons)/m².s,11-100%可调
光源:标准配置蓝光455nm,可根据需求配备不同波长的LED光源
检测器:PIN光电二极管,667–750nm滤波器
尺寸大小:超便携,手机大小,134×65×33mm(不包括探头),重量仅188g
数据存储:容量16Mb,可存储149000数据点
显示与操作:图形化显示,双键操作,待机5分钟自动关闭
供电:2000mA可充电锂电池,USB充电,可连续工作48小时,低电报警
工作条件:0–55℃,0–95%相对湿度(无凝结水)
存贮条件:-10–60℃,0–95%相对湿度(无凝结水)
通讯方式:蓝牙+USB双通讯模式,蓝牙在20m距离ZD传输速度3Mbps
GPS模块:内置,ZG精度1.5m
软件:FluorPen1.1专用软件,用于数据下载、分析和图表显示,输出Excel数据文件及荧光动力学曲线图,适用于Windows 7及更高操作系统
操作软件与实验结果
产地:捷克
应用案例:

2017年4月,美国航空航天局(NASA)新一代先进植物培养器(Advanced Plant Habitat,APH)搭载联盟号MS-04货运飞船抵达国际空间站。宇航员使用FluorPen手持仪叶绿素荧光仪在其中开展植物生理学及太空食物种植(growth of fresh food in space)的研究。
参考文献
1. Singh, S., Mohan Prasad, S. & Pratap Singh, V. Additional calcium and sulfur manages hexavalent chromium toxicity in Solanum lycopersicum L. and Solanum melongena L. seedlings by involving nitric oxide. Journal of Hazardous Materials 398, 122607 (2020).
2. Ariyarathna, R. a. I. S., Weerasena, S. L. & Beneragama, C. K. Application of Polyphasic OJIP Chlorophyll Fluorescent Transient Analysis as an Indicator for Testing of Seedling Vigour of Common Bean (Phaseolus vulgaris L.). Tropical Agricultural Research 31, 106–115 (2020).
3. Prity, S. A. et al. Arbuscular mycorrhizal fungi mitigate Fe deficiency symptoms in sorghum through phytosiderophore-mediated Fe mobilization and restoration of redox status. Protoplasma (2020) doi:10.1007/s00709-020-01517-w.
4. Rahman, M. A. et al. Arbuscular Mycorrhizal Symbiosis Mitigates Iron (Fe)-Deficiency Retardation in Alfalfa (Medicago sativa L.) Through the Enhancement of Fe Accumulation and Sulfur-Assisted Antioxidant Defense. International Journal of Molecular Sciences 21, 2219 (2020).
5. Vitorino, L. C. et al. Biocontrol Potential of Sclerotinia sclerotiorum and Physiological Changes in Soybean in Response to Butia archeri Palm Rhizobacteria. Plants 9, 64 (2020).
6. Aalifar, M. et al. Blue Light Improves Vase Life of Carnation Cut Flowers Through Its Effect on the Antioxidant Defense System. Front. Plant Sci. 11, 511 (2020).
7. Muthusamy, M., Yoon, E. K., Kim, J. A., Jeong, M.-J. & Lee, S. I. Brassica Rapa SR45a Regulates Drought Tolerance via the Alternative Splicing of Target Genes. Genes 11, 182 (2020).
8. Muthusamy, M., Kim, J. Y., Yoon, E. K., Kim, J. A. & Lee, S. I. BrEXLB1, a Brassica rapa Expansin-Like B1 Gene Is Associated with Root Development, Drought Stress Response, and Seed Germination. Genes 11, 404 (2020).
9. Herritt, M. T. & Fritschi, F. B. Characterization of Photosynthetic Phenotypes and Chloroplast Ultrastructural Changes of Soybean (Glycine max) in Response to Elevated Air Temperatures. Front. Plant Sci. 11, (2020).
10.Kasampalis, D. S., Tsouvaltzis, P. & Siomos, A. S. Chlorophyll fluorescence, non-photochemical quenching and light harvesting complex as alternatives to color measurement, in classifying tomato fruit according to their maturity stage at harvest and in monitoring postharvest ripening during storage. Postharvest Biology and Technology 161, 111036 (2020).
11.Soares, J. S., Santiago, E. F. & Sorgato, J. C. Conservation of Schomburgkia crispa Lindl. (Orchidaceae) by reintroduction into a fragment of the Brazilian Cerrado. Journal for Nature Conservation 53, 125754 (2020).
12.Poblete, T. et al. Detection of Xylella fastidiosa infection symptoms with airborne multispectral and thermal imagery: Assessing bandset reduction performance from hyperspectral analysis. ISPRS Journal of Photogrammetry and Remote Sensing 162, 27–40 (2020).
13.Chiluwal, A. et al. Deterioration of ovary plays a key role in heat stress-induced spikelet sterility in sorghum. Plant, Cell & Environment 43, 448–462 (2020).
14.Maai, E., Nishimura, K., Takisawa, R. & Nakazaki, T. Diurnal changes in chloroplast positioning and photosynthetic traits of C4 grass finger millet. Plant Production Science 0, 1–13 (2020).
15.De Micco, V. et al. Dust accumulation due to anthropogenic impact induces anatomical and photochemical changes in leaves of Centranthus ruber growing on the slope of the Vesuvius volcano. Plant Biol J 22, 93–102 (2020).
附:OJIP参数及计算公式
Bckg = background
Fo: = F50µs; fluorescence intensity at 50 µs
Fj: = fluorescence intensity at j-step (at 2 ms)
Fi: = fluorescence intensity at i-step (at 60 ms)
Fm: = maximal fluorescence intensity
Fv: = Fm - Fo (maximal variable fluorescence)
Vj = (Fj - Fo) / (Fm - Fo)
Fm / Fo = Fm / Fo
Fv / Fo = Fv / Fo
Fv / Fm = Fv / Fm
Mo = TRo / RC - ETo / RC
Area = area between fluorescence curve and Fm
Sm = area / Fm - Fo (multiple turn-over)
Ss = the smallest Sm turn-over (single turn-over)
N = Sm . Mo . (I / Vj) turn-over number QA
Phi_Po = (I - Fo) / Fm (or Fv / Fm)
Phi_o = I - Vj
Phi_Eo = (I - Fo / Fm) . Phi_o
Phi_Do = 1 - Phi_Po - (Fo / Fm)
Phi_Pav = Phi_Po - (Sm / tFM); tFM = time to reach Fm (in ms)
ABS / RC = Mo . (I / Vj) . (I / Phi_Po)
TRo / RC = Mo . (I / Vj)
ETo / RC = Mo . (I / Vj) . Phi_o)
DIo / RC = (ABS / RC) - (TRo / RC)
